Is Matter around us Pure Class 9 Science
Chapter-2
Is Matter Around us Pure
Substance
A substance may be defined as a pure single form of matter.
Matter may be classified into two kinds of substances
– Pure substances and Mixtures
Pure substance
A substance that consists of just one sort of only particle is known as a Pure Substance.
As Examples, Diamond, Salt, Sulfur, Tin.
Mixture
Mixtures are the combination of various substances. As an Example, Lemonade, it is a mixture of three substances,i.e. Lemon Juice, Sugar and Water.
Few examples:
Mixture – Cake, Soil, Air
Pure substance – Water, Copper, Hydrogen, etc.
Types of Mixtures
There are mainly two kinds of mixtures:
Homogeneous Mixtures and
Heterogeneous Mixtures
Homogenous Mixtures
When we add sugar, water and lemon juice with each other, all of them uniformly combine with one another. Now it is impossible to separate these substances from the new mixture. Such mixtures in which the various elements combine with each other uniformly are referred to as Homogeneous Mixtures.
• The ratio of compositions of homogeneous mixtures may be totally different. For Example, one might add two spoons of sugar in lemonade while somebody else might add only one spoon of sugar in their lemonade. But in both situations, the lemonade is a homogeneous mixture.
Heterogeneous Mixtures
The elements in a heterogeneous mixture don't fully dissolve in one another and we may separate them by physical methods. Hence, we can say that the composition of such mixtures is not uniform at all.
As an Example, If we combine sand in water, then the sand settles down in water after some time and we may separate it by a method, i.e. by filtration.
Here are a few differences between homogeneous and heterogeneous mixtures –
Solution
A solution is a uniform combination or mixture of two or more substances. Homogenous Mixtures are the examples of solutions.
Types of Solution
Solutions of -
• Liquid into liquid: e.g. Water and Ink
• Solid into solid: e.g. Alloys
• Gas into gas: e.g. Air
• Solid into liquid: e.g. Sugar and Water
• Solid into gas: e.g. Hydrogen and Metals
• Liquid into gas: e.g. Carbon Dioxide and Water
Alloy
An alloy is a combination or mixture of different metals or non-metals and metals, that cannot be separated easily from each other by using simple physical methods. For Example:
Brass – mixture of Copper with up to 50% zinc
Bronze – mixture of Copper with up to 12% tin
Solvent and Solute
A Solution consists of two types of substances or components, called solute and solvent.
Solution = Solute + Solvent
Solvent – The substance in which another substance or element is added is called a Solvent. For Example, in a solution of water and salt, Water is a solvent in which salt is added.
Solute – The substance that is added into the solvent to make a solution is called a Solute. For Example, in the solution of water and Salt, water is a solvent while salt is a solute which is added into the solvent to make a solution.
Properties of a Solution:
A solution is simply a homogeneous mixture.
We can't see the particles of a solution through naked eyes as they are so small in size, about 1 nanometer in diameter.
The path of light cannot be visible through the solution.
The particles of a solution can not scatter light through them because they are extremely small in size.
We can't separate the particles of a solution by methods of filtration.
Stable Solution
A stable solution is a solution, whose particles do not settle down when we leave it undisturbed for some time, because the particles of a stable solution are homogeneously spread.
Different Types of Solutions
• Dilute :–
A solution is said to be dilute if the concentration of the solute is much lesser than that of the concentration of solvent.
For Example, If we mix 1gm of salt in 500 ml of water, the solution obtained will be diluted, as the amount of solute is much lesser than the solvent. If we keep on adding the solute in a solution there comes a point when no more solute dissolves in the solution. This is called the Saturation Point of a Solution.
• Unsaturated Solution :–
A solution is said to be Unsaturated, if we can add more amount of solute as it has not achieved its saturation level yet. A dilute solution can be considered as an Unsaturated Solution.
• Concentrated Solution :–
A solution with a large amount of solvent is referred to a Concentrated Solution.
• Saturated Solution :–
A solution in which no more solute can be added because it has already dissolved the maximum amount of solute it can, is called a Saturated Solution.
Concentration
Concentration is referred to as the amount of a substance per defined space. It can be defined as the ratio of solute in a solution to either solvent or total solution.
To calculate the concentration, we can use the formulae below:
• Percent by Mass = (Mass of solute / Mass of solution) X 100
• Percent by Volume = (Volume of solute / Volume of solution) X 100
Suspension
A suspension is formed when two or more substances are mixed in a non-uniform manner. Heterogeneous mixtures are the examples of suspensions. The solute does not mix with the solvent and can be viewed through naked eyes.
Properties of Suspensions:
• A suspension is a heterogeneous mixture.
• We can see the particles of suspensions through naked eyes.
• We can see the path of light through the particles of a suspension.
• The particles of suspension always tend to settle down when it is left undisturbed. And hence, they can be separated using filtration.
Colloids or Colloidal Solutions
A colloidal solution or a colloid is referred to as a uniform solution of two or more substances. The particles are relatively very small in size that the solution appears as a homogeneous mixture but it is not.
Properties of colloids:
• Colloids or colloidal solutions are heterogeneous in nature.
• The particles of a colloid can't be seen through naked eyes.
• The particles can scatter the beam of light passed through a colloid and hence produce Tyndall effect.
• Colloids are stable in nature. The particles of colloids don't settle down when they are left undisturbed.
• We can't separate the particles of a colloid or Colloidal Solution through the filtration method. We use another method called Centrifugation to separate the particles of a colloid.
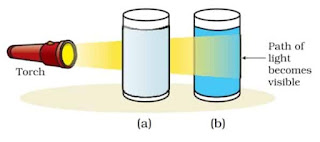
Tyndall Effect
When a beam of light passes through a colloidal solution or colloid, the particles of the colloid scatter the beam of light and hence we can see the path of light in the solution. This phenomenon is known as the Tyndall effect.
For Example, when a ray of light enters a dark room it is scattered by the dust particles present in the air and we see the path of light clearly.
 |
| Tyndall Effect |
Separating the Components of a Mixture
heterogeneous mixtures can be separated into their constituents by means of simple physical methods like:
• Filtration
• Hand-picking
• Sieving
The components of a mixture can also be separated from each other by using several other techniques like:
• Evaporation
• Centrifugation
• Sublimation
• Chromatography
• Distillation
1. Evaporation
we use the Evaporation method for separating a mixture of a non-volatile and a volatile substance.
• Applications:
For Separating coloured component from the ink
Salt from water
Sugar from Water
• Method:
We heat a beaker, halfly filled with water and then put a watch glass on the top of the beaker and then put a few drops of ink on it. The process of Evaporation can be seen through the ink leaving behind the coloured substance.
2. Centrifugation
Centrifugation method is used in Separating dense particles from lighter particles.
Applications:
Separating milk from cream
Separating butter from cream
Squeezing out water from wet clothes
• Method:
Milk is put in a centrifuging machine for two minutes or in milk churner and the cream thus separates from milk.
3. Separating funnel
It is used to separate two immiscible liquids.
• Applications:
o To separate Oil from water.
o In the extraction of Iron from its ore.
Method:
The immiscible liquids are made to settle in the funnel and after some time, they form separate layers due to varying densities. The lower most liquid is allowed to flow out of the funnel and after it is completely poured out, the stopcock is closed thereby separating the two liquids from each other.
4. Sublimation
This method is used to separate a sublimable component from a non-sublimable component.
• Applications:
o Ammonium chloride / camphor / naphthalene and salt
• Method:
In his method, We heat the mixture in an inverted funnel so that the sublimable component sublimes in the air and fixes over the walls of the funnel and the non-sublimable component, on the other hand, is left behind on the bottom of the inverted funnel.
5. Chromatography
This method is used to separate solutes that can dissolve in the same solvent.
• Applications:
o For Separating colour components of a dye
o To Separate Drugs from blood
• Method:
In this method, we take a filter paper or a blotting paper and place a drop of ink at the rear end. Then we dip that end in water. As ink is a mixture of two or more colors, the component of ink which is soluble in water mixes into it and hence separates quickly from the other components that are less soluble in water.
6. Distillation
It is used to separate miscible liquids (the boiling points of the liquids must be sufficiently different).
Applications:
o To Separate Acetone and water
• Method:
o The mixture is heated in a distillation apparatus. The substance with lower boiling point evaporates first, condenses and gets separated from the other substance with a higher boiling point.
o Simple Distillation
when the miscible liquids have a satisfactory difference in their boiling points.
o Fractional Distillation
when the difference between the boiling points of the liquids is less than 25 K.
Separating different Gases from the Air by using Fractional Distillation Method
First, we compress and cool the air by increasing the temperature and decreasing the pressure. The air turns to liquid air.
Liquid air is then warmed up slowly in a fractional distillation apparatus.
After a while, the several components of air get separated and are collected at various heights on the basis of their boiling points.
Crystallization
It is used in Purifying Solids.
In this method, we can get a pure solid in the form of crystals from its solution.
Applications:
o Salt from sea water
o Purification of copper sulphate
• Method:
o The impurities of a substance are filtered out.
o Water is evaporated to obtain a saturated solution.
o The solution is covered with filter paper and left as it is.
o After some time, the crystals of pure solid are formed.
• Is evaporation better than crystallization?
Simple evaporation is not better than crystallization
because:
1. In evaporation, some solid substances often decompose because of the excess heat.
For Example, Sugar gets charred on extra
heating.
2. If after the filtration method, some impurities remain in the solution then they can contaminate the solid and therefore we would not obtain a pure substance.
Physical Change and Chemical Change
Physical Property :
The Properties of a substance such as rigidity, colour, fluidity, boiling point, melting point, density and hardness which we can observe are called Physical Properties.
Physical Change:
Whenever the physical properties of a substance change, it is termed as a Physical Change. As an example, When we convert a substance from one state to another, such as a solid into a liquid or vice-versa, it is also a physical change because only the physical nature of the substance changes in this process without disturbing its chemical nature.
For Example, Change of ice into water. During this change in state, The chemical properties of water remain the same.
Chemical Property:
The chemical behaviour or nature of a substance is known as its Chemical Property, for example, its odour or its chemical composition.
Chemical Change:
When the chemical properties or chemical composition of a substance gets changed, it is called a chemical change. It is also known as a Chemical Reaction.
For Example, Burning of paper
Types of Pure Substances
Pure substances are classified as
elements and
compounds
Elements
An element is the simplest form of matter. Elements cannot be broken down into further elements by chemical reactions.
Elements are further characterized as
Metals,
Non-Metals and
Metalloids
Metals – Silver, Mercury, Copper, Gold
1. Metals are lustrous (shiny)
2. Metals conduct heat and electricity
3. Metals have a silver-grey or gold-yellow colour
4. We can hammer metals and form thin sheets
(Malleability)
5. We can convert metals into wires (Ductility)
6. Metals always produce a ringing sound if they are hit (Sonorous).
Non-Metals – Carbon, Iodine, Chlorine, Oxygen, Hydrogen
1. Non-Metals do not conduct heat and electricity
2. Non-Metals are not sonorous, lustrous or ductile
3. Non-Metals have varied colours
Metalloids – Silicon, Germanium
They show some properties of metals and some of the non-metals.
Quick Facts –
1. There are 100 elements known to us
2. 92 elements out of them occur naturally
3. Rest, 8 are man-made elements
4. Most of the elements are solid in nature
5. At room temperature, 11 elements exist in the gaseous state
6. At room temperature, 2 elements exist in the liquid state – bromine and mercury
7. At a temperature slightly higher than room temperature, 2 elements exist in the liquid state –calcium and gallium.
Compounds
It is a substance that consists of two or more substances. These substances are combined chemically with each other in fixed proportions to form a compound. The properties of
compounds are different from that of its constituents. For Example, Ammonium Sulphate, Sulphur Chloride, Water, etc.
Difference between Mixture and Compounds
Mixtures vs. Compounds
Click to get notes of :




0 Comments
If you have any doubt, please let me know. Leave a comment here...